Reopening America: COVID-19 Vaccine Update
FDA Establishes Guidelines
As the global effort to combat the COVID-19 pandemic continues, the FDA has taken action to help facilitate the timely development of a vaccine. Vaccines can typically take years to develop, however there has been a global effort to expedite the development of a COVID-19 vaccine due to the widespread and unpredictable nature of the virus. In a statement released on June 30th, FDA Commissioner Stephen M Hahn, M.D. said “We recognize the urgent need to develop a safe and effective vaccine to prevent COVID-19 and continue to work collaboratively with industry, researchers, as well as federal, domestic, and international partners to accelerate these efforts. While the FDA is committed to expediting this work, we will not cut corners in our decisions and are making clear through this guidance what data should be submitted to meet our regulatory standards. This is particularly important, as we know that some people are skeptical of vaccine development efforts.” In these guidelines, the FDA emphasizes the importance of various typical testing protocols such as a large sample size and diverse population. While these guidelines are not necessarily full of new information for drug developers, they are still important as they emphasize that the FDA is not sacrificing safety for the sake of speed.
The Vaccine Testing Process
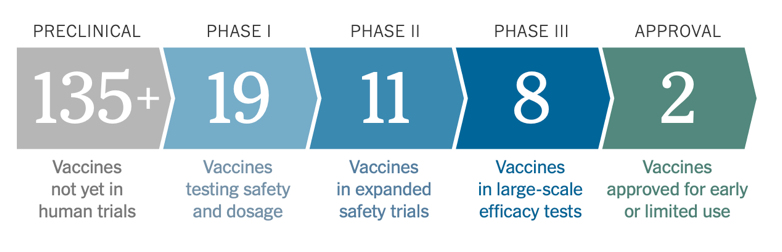
There are 4 stages a vaccine must pass through before being approved. The first is preclinical testing which consists of scientists administering the proposed vaccine to animals to see how their immune system reacts. Assuming positive results, the drug then moves into 3 phases of human trials.
Phase I is safety trials. Scientists give the vaccine to a small number of people to confirm that what they saw in the animal trials holds true in humans. They also test different, dosages to find the optimal amount of the drug to be administered further down the line. Next comes Phase II which consists of expanded trials. The vaccine is administered to hundreds of people of all different age groups to further evaluate the drug’s effectiveness. The final phase before approval is efficacy trials. In this stage, the vaccine is administered to thousands of people. The infection rate of those who receive the vaccine is directly compared to a placebo group. This phase is particularly crucial, as it ensures the effectiveness of the vaccine and not just the safety. In order for a COVID-19 vaccine to be considered effective, the FDA has determined that it will need to protect at least 50% of vaccinated people.
After a vaccine travels through all of these phases of testing, regulators review the trial results and decide whether to approve the vaccine or not. In dire situations, such as the current pandemic, phases can be combined for accelerated vaccine development. Most commonly, Phases I & II are combined resulting in the drug being tested for the first time on hundreds of people.
Hope for the Future
Parts of the country are now experiencing a new surge in COVID-19 cases as a direct result of reopening the country. With many businesses that are capable of working remotely telling employees that they have no intention of bringing them back into the office until there is a vaccine, many are left wondering when exactly a vaccine will hit the market and we will be able to return to life as we once knew it. According to The New York Times Coronavirus Vaccine Tracker, there are currently more than 165 vaccines currently being developed worldwide. The below graphic shows the phase in which each vaccine is as of August 11th, 2020.
While the graphic shows that 2 vaccines have been approved for early or limited use, neither of these drugs have been approved in the United States. There are, however, two drugs that are currently being developed and tested in the United States. The first is being developed by the German BioNTech in conjunction with New York based Pfizer and Chinese drug maker Fosun Pharma. The company announced on July 27 th that it would launch its Phase II/III trial with around 30,000 volunteers in Argentina, Brazil, Germany, and the United States. The Trump administration awarded a $1.9 billion contract for 100 million doses to be delivered by December with the option to acquire 500 million more doses.
The second , and most promising, is being developed by Moderna, a U.S. based company which partnered with the National Institutes of Health. Moderna put the first COVID-19 vaccine into human trials. The company moved into the third and final phase of testing on July 27 th , giving prominent officials such as Dr. Anthony Fauci hope for the future. The government as financially supported much of Moderna’s efforts, investing over one billion dollars. While there are no guarantees, the government hopes to have results and a vaccine in place by the end of the year.
